TromboneAl
Give me a museum and I'll fill it. (Picasso) Give me a forum ...
- Joined
- Jun 30, 2006
- Messages
- 12,880
I came across this product
Battery Xtender
which claims to recharge disposable alkaline batteries (info and review) up to about 10 times.
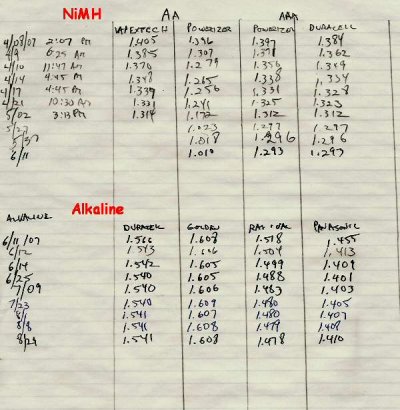
Since 2005, California has required recycling of alkaline batteries, so I've been putting my used batteries in a box. When I read about the charger, I thought I'd see how many I had for recharging. Turns out that even though I already use NiMH rechargeables for most things, I still had about 80 alkalines waiting to be recycled.
The main problem with the NiMH batteries is that they self-discharge. That is, if you leave it in a box, or use it for a remote control, it will go from 1.4V to 1.0 V in a month or so. So for very low drain applications, or for something that sits a long time between use (emergency flashlight), alkaline is better. Also, my NiMH charger doesn't work with C or D sized batteries.
I've been going through the box of used batteries, and am able to recharge most of them. Here is an example of some pre and post-charge voltages:
Pre Post
.185 can't charge (dead)
1.484 1.486
1.263 1.484
1.282 1.485
1.539 1.570
1.368 1.521
1.443 1.445
1.058 1.304
0.370 1.347
1.469 1.556
1.094 1.480
Battery Xtender
which claims to recharge disposable alkaline batteries (info and review) up to about 10 times.
Since 2005, California has required recycling of alkaline batteries, so I've been putting my used batteries in a box. When I read about the charger, I thought I'd see how many I had for recharging. Turns out that even though I already use NiMH rechargeables for most things, I still had about 80 alkalines waiting to be recycled.
The main problem with the NiMH batteries is that they self-discharge. That is, if you leave it in a box, or use it for a remote control, it will go from 1.4V to 1.0 V in a month or so. So for very low drain applications, or for something that sits a long time between use (emergency flashlight), alkaline is better. Also, my NiMH charger doesn't work with C or D sized batteries.
I've been going through the box of used batteries, and am able to recharge most of them. Here is an example of some pre and post-charge voltages:
Pre Post
.185 can't charge (dead)
1.484 1.486
1.263 1.484
1.282 1.485
1.539 1.570
1.368 1.521
1.443 1.445
1.058 1.304
0.370 1.347
1.469 1.556
1.094 1.480