Chuckanut
Give me a museum and I'll fill it. (Picasso) Give me a forum ...
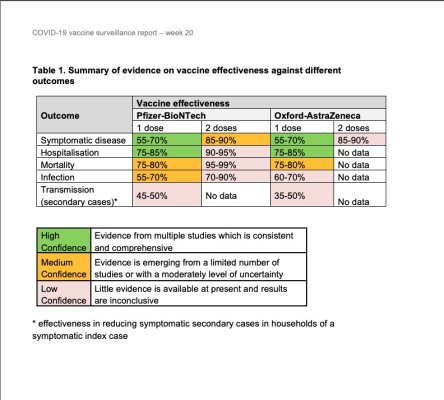
Here's a paper published by Public Health England regarding the effectiveness of two vaccines (Pfizer-BioNTeach and Astra Zenica) and their effect on the population.
https://assets.publishing.service.g...193/Vaccine_surveillance_report_-_week_20.pdf
It appears to be a compilation of various studies and trials from December 8 to May 16.
Note: Table 1. It's very interesting.
Going through the document confirms that while the vaccines are very effective in some areas, there are areas that need further study. More information is needed to increase the level of certainty. The authors appear to be taking a very conservative approach to evaluating the vaccines. I like that careful approach.
https://assets.publishing.service.g...193/Vaccine_surveillance_report_-_week_20.pdf
It appears to be a compilation of various studies and trials from December 8 to May 16.
The data in this week’s report covers the period from 8 December 2020 to 16 May 2021(Figure 5). It shows the provisional number and percentage of people in England who have had received 1dose or 2 doses of a COVID-19 vaccination by age group and week since the start of the programme.
Compared to unvaccinated, vaccine effectiveness for 1dose was estimated at 54% (95% CI 50-58%) for the Pfizer-BioNTech vaccine and 53% (95%CI 49-57%) for the Oxford-AstraZeneca vaccine. Compared to 4to 13 days post vaccination, this was 57% (95%CI 53-61%) and 58% (95%CI 54-62%) respectively.
After 2 doses, effectiveness was estimated as 90% (95%CI 82-95%) for the Pfizer-BioNTech vaccine and 89% (95%CI 78-94%) for the Oxford-AstraZeneca vaccine compared to unvaccinated. Compared to 4to13 days post vaccination,this was 91% (95%CI 83-95%) and 90% (95%CI 80-95%) respectively.
With the Pfizer-BioNTech vaccine there is a small reduction in vaccine effectiveness from 10 weeks after the first dose. This may be explained by some waning of protection or by biases due to differences in the earliest groups who were vaccinated compared to later groups. There is no evidence of this waning effect with the Oxford-AstraZeneca vaccine.
Note: Table 1. It's very interesting.
Going through the document confirms that while the vaccines are very effective in some areas, there are areas that need further study. More information is needed to increase the level of certainty. The authors appear to be taking a very conservative approach to evaluating the vaccines. I like that careful approach.